NeoCare in Congenital Heart Defects
NeoCare is a medical device developed for newborns affected by severe heart defects
Thanks to public and philantropic funding, NeoCare has been designed to adjust the blood flow going to the heart of newborn, in particular for premature babies with severe heart defects who are not in a condition to have reparative surgery at birth.
About Congenital Heart Defects
Present at birth, Congenital Heart Defects (CHD) are structural malformations of the heart that trigger a wide range of crippling cardiac dysfunctions.
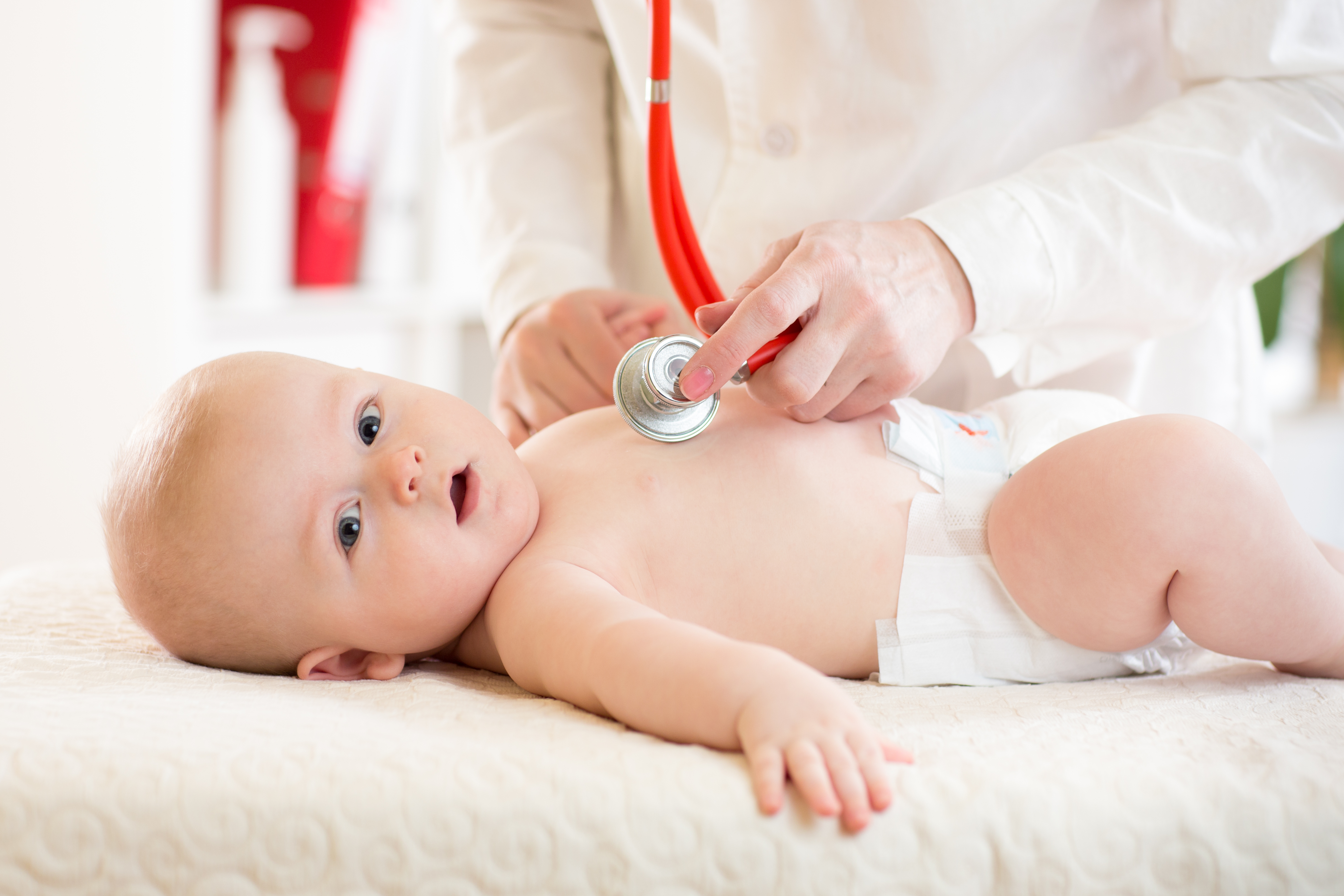
Congenital Heart Defects appear in 8 / 1000 newborns worldwide. When available, early corrective heart surgery is the preferred intervention to repair the defects. In some cases, this open-heart surgical operation on a very small heart is not an option and a palliative and temporary procedure is performed instead.
One such common palliative procedure is known as Pulmonary Artery Banding (PAB) and is currently used in the USA and Europe in about 1000 cases per year. PAB involves suturing a fixed length of an implantable ‘tape’ around the pulmonary artery to narrow the latter. This device allows to tamper down the abnormal blood pressure and thereby protects the heart and lung functions of these babies. This buys newborns and doctors precious time before these babies can undergo the final corrective open-heart surgical procedure.
Unfortunately, conventional PAB is far from being optimal. The implantation of the band is conducted under general anaesthesia and artificial respiration which makes the adjustment of the device very difficult, so that long stays in intensive care and further operations to re-adjust the band tightness are often required. This situation is traumatic to newborn babies and their parents and results in increased mortality and morbidity. This problem is even more acute in developing countries where surgery and post-operative care are often basic at best.
NeoCare aims at overcoming the challenges of conventional PAB
This miniature, implantable, and battery free device is suited for banding small size arteries, thus performing non-invasive adjustments of pulmonary arteries of newborns and children with heart defects. The technology comprises an implant coupled with an external control unit; the latter allows after implantation to remotely control and regulate the blood flow without having to open the chest. It results in shorter stays in intensive care and no-need for re-operations to adjust the banding and the flow in the pulmonary artery.
In collaboration with the School of Management and Engineering Vaud (HEIG-VD), the cybersecurity protection software is under deelopment to be included in the device. HEIG-VD is using its expertise to develop prototypes to best achieve clinical requirements for an efficient and safe PAB in neonates born with congenital heart defects.
EspeRare is looking for partners to externalise the manufactuirng of the device which will enable to reach a broad number of patients in particular premature neaonates who cannot tolerate an open chest surgery at birth.
Key achievements for NeoCare PAB device
- > Small diameter and size to better fit to neonates anatomy of premature babies

- > Variable size for PAB, according to the therapeutic indication
- > Deported antenna to improve energy transmission efficiency
- > Can be indicated for babies < 1kg and > 8 kg
> Long term implantation
> User interface, IT security, data protection


